Natural Water to Hydrogen
The Natural Water to Hydrogen research profile is an initiative of the University of Duisburg-Essen (UDE) with the aim of increasing the sustainability of hydrogen (H2)-production via water electrolysis.
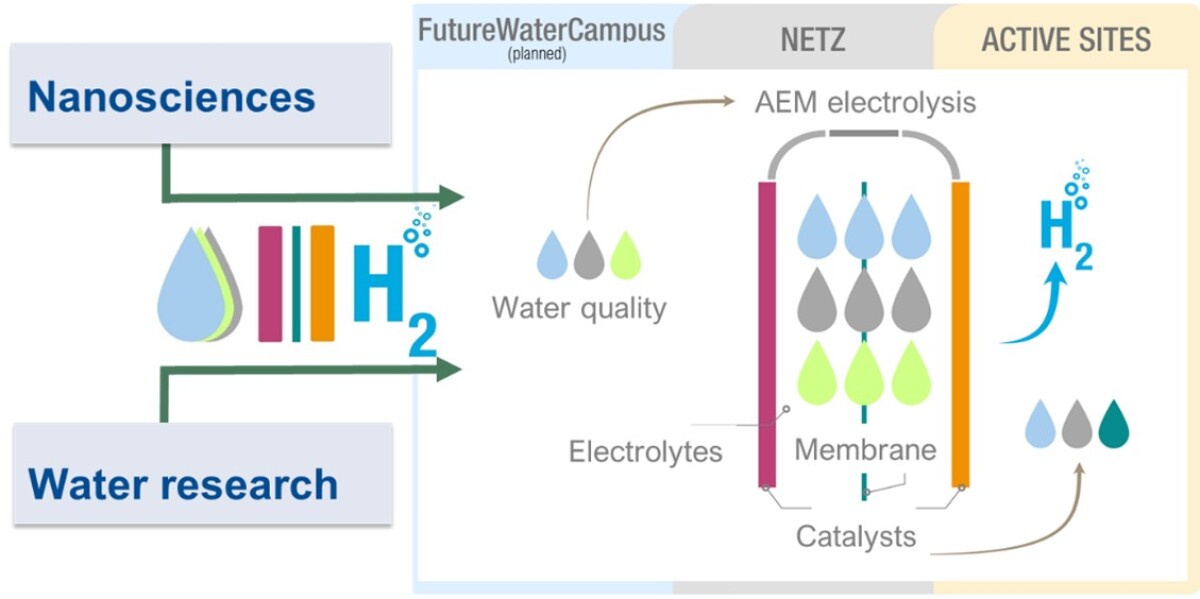
In this profiling project, the influence of various water components - in addition to typical anions and cations, also organic substances occurring in natural waters – will be investigated on the efficiency and long-term stability of the electrolysis process is. We are focussing on low-temperature anion exchange membrane (AEM) electrolysis. AEM electrolysis is a fluorine-free technology that can be operated with naturally occurring electrocatalysts that are not based on rare precious metals and offers significant advantages over established proton exchange membrane (PEM) electrolysers in terms of cost and scalability. The central aim of the research project is to define the water quality that enables efficient and robust AEM electrolysis with established catalysts and membranes.
In fundamental, application-orientated studies, the operation of the electrolysers is simulated in order to determine the effects of the various water components on AEM electrolysis. Knowledge of the actual requirements for the optimum or minimum required water quality for AEM electrolysis enables the use of only minimally treated water and also defines the water quality to be maintained during electrolysis.
The new research profile will bundle the UDE strategic research area of water research and nanosciences (catalysis) in the thematic field of "Natural Water to Hydrogen". This new approach of systematically linking water research with electrochemistry and membrane / material science will drive excellent, innovative and future-oriented research on H2 electrolysis from renewable energies and contribute to the provision of sustainable energy resources for future generations.
Spokeperson
Prof. Dr.-Ing. Corina Andronescu, Technical Chemistry III - Electrochemical Catalysis


