Synthesis in ionic liquids
4.3.2 Synthesis in ionic liquids
Ionic liquids are organic salts with a melting point below 100 °C. In general they consist of a bulky organic cation and a weakly-coordinating anion (figure 1). This leads to different properties from classical solvents, like a negligible vapor pressure. Electrostatic interactions between particle surface and ionic liquid stabilize nanoparticles, which renders surface modifying agents unnecessary.
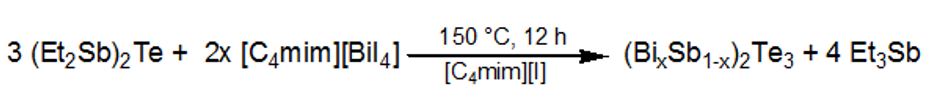
ILs are thermally stable at high temperatures and are ideal solvents for microwave reactions due to their high polarisability and conductivity. In our current researches ionic liquids are used both as a solvent as well as a reactive reagent. For instance, [C4mim][BiI4] was prepared by reactioon of [C4mim]I with BiI3 and structurally characterized by single crystal X-ray diffraction using the in situ crystallization method.
The combination of metal-containing ionic liquids and metal-organic precursors allows the synthesis of various materials. For instance, Bi2Te3 was successfully synthesized by thermolysis of bis(triethylsilyl)telluride in a mixture of 1-butyle-3-methyle-imidazolium iodide ([C4mim][I]) and 1-butyle-3-methyle-imidazolium tetraiododbismuthate ([C4mim][BiI4]).
The phase purity of the materials was proven by PXRD, while the composition was verified by EDX and AES. XPS studies and IR studies clearly showed thatthe material do not conatin any IL on the surface.
In addition to the synthesis of group 15/16 materials, metal halide anions such as CoCl3- or SbCl4- are investigated as precursors for the synthesis of binary intermetallic materials including nanoparticular thermoelectric materials.
References
[1] K. Richter et al., Phys. Status Solid B 2013, 6, 1152
[2] M. Antonietti et al., Angew. Chem. Int. Ed. 2004, 43, 4988
[3] J. Dupont et al., Chem. Rev. 2002, 102, 3667
[4] C. Janiak, Z.Naturforsch. 2013, 68b, 1059-1089
Go back: 4.3.1.3 Group 15 Chalcogenides