Nanowires
GaSb nanowires
The simple pyrolysis of GaSb-adducts of type R3GaSbR'3 in sealed glass ampoules in the temperature range 350-500 °C yields GaSb crystallites.[1] Their carbon content is below the detection limit of the electron energy loss spectroscopy (EELS), while oxygen is present as a surface contamination.
Dendritic-like growth was observed at high decomposition temperatures while isolated GaSb nanowires were rather obtained at lower temperatures (250 - 350 °C) as well as from other adducts such as [t-Bu3Ga]2[Sb2Et4]. The nanowires were characterized in detail by EDX, EELS (Sb-mapping) and high-resolution transmission-electron-microscopy (HR-TEM).[2]
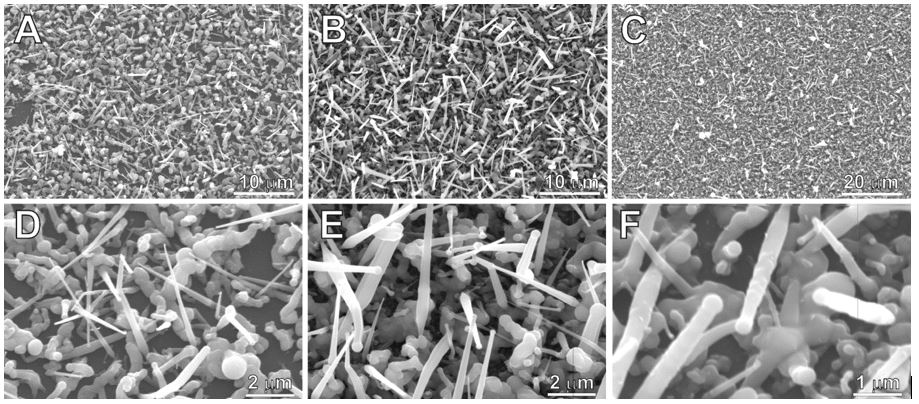
Figure 2: SEM images showing GaSb dendrites as-obtained from (t-Bu)3Ga–Sb(i-Pr)3 at Tdec. = 275 °C (A, D), 300 °C (B, E), and 325 °C (C, F).
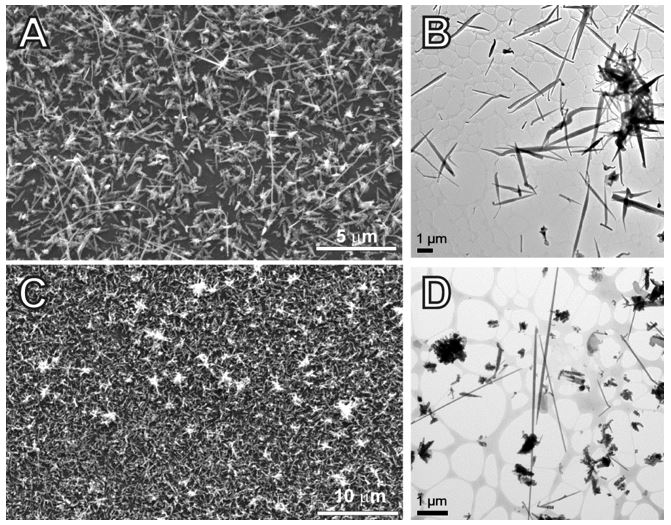
Figure 3. SEM and TEM images of GaSb nanowires obtained from [t-Bu3Ga]2[Sb2Et4] at Tdec. = 250 °C (top) and 400 °C (bottom). D shows the formation of GaSb nanowires with high aspect ratio.

Figure 4. SAED pattern with simulation as an overlay and HRTEM image of of GaSb nanowires obtained from [t-Bu3Ga]2[Sb2Et4] at Tdec. = 250 °C in <110> zone axis. The indicated distances of 353 and 305 pm correspond to {111} and {200} lattice fringes, respectively.
In addition, a rather controlled self-catalyzed growth of GaSb nanowires using 90 nm-sized Ga droplets, which were pre-deposited on Si(100) substrates by thermal decomposition of t-Bu3Ga, was achieved with Sb2Et4 at 250 °C.[2] Ga droplets have been shown in the past to initiate nanowire growth of a variety of materials such as Si and Ge.[3] The pyrolysis experiments clearly showed that Ga droplets act as an initiator (catalyst) for the GaSb nanowire growth and the growth of the nanowires, which occured according to the so-called VLS mechanism (vapor-liquid-solid),[4] showed a strong dependency on the pyrolysis temperature.
The decomposition of t-Bu3Ga forms a Ga-droplet on the substrate, which catalyse the decomposition of the Sb-precursor, resulting in an enrichment of the Ga-droplet with elemental Sb. After exceeding the saturated solution level, the crystallization starts at the liquid-solid interface and the nanowire is formed and the Ga-droplet is preserved on the top of the whisker.
Bismuth nanowires
Bismuth is a semimetal, promising very large quantum size effects due to the small effective mass of charge carriers and very low carrier density.[5] Bi-nanowires, were predicted to undergo a transition from semimetal to semiconductor with diameters smaller than 50 nm (77 K), or <10 nm (25 °C). In addition, Bi-nanowires are discussed as interesting thermoelectric materials and different procedures for the production of nanowires and nanocrystals have been developed.[6] However, typical procedures such as hydrothermal methods,[7] particularly electrochemical deposition in porous aluminium oxide templates and others,[8,9] typically bares problems related to the low melting point of Bi (271.3 °C).
In the course of our studies we focused our investigations on the deposition of Bi-nanowires by MOCVD process. Bi nanowires were obtained with trialkylbismuthanes as well as dibismuthanes under very mild reaction conditions, below 200 °C using Bi2Et4.
References
[1] A. Kuczkowski, S. Schulz, W. Assenmacher, J. Mater. Chem. 2001, 11, 3241.
[2] S. Schulz, M. Schwartz, A. Kuczkowski, W. Assenmacher, J. Cryst. Growth 2010, 312, 1475.
[3] H. Chandrasekaran, G. U. Sumanasekara, M. K. Sunkara, J. Phys. Chem. B 2006, 110, 18351.
[4] R. S. Wagner, W. C. Ellis, Appl. Phys. Lett. 1964, 4, 89.
[5] a) S. B. Cronin, Y.-M. Lin, O. Rabin, M. R. Black, J. Y. Ying, M. S Dresselhaus, P. L. Gai, J.-P. Minet, J.-P. Issi, Nanotechnol. 2002, 13, 653. b) N. Garcia, Y. H. Kao, M. Strongin, Phys. Rev. B 1972, 5, 2029.
[6] a) J. Heremans, C. M. Thrush, Phys. Rev. B 1999, 59, 12579; b) Y. M. Lin, X. Z. Sun, M. S. Dresselhaus, Phys. Rev. B 2000, 62, 4610; c) Y.-M. Lin, O. Rabin, S. B. Cronin, J. Y. Ying, M. S Dresselhaus, Appl. Phys. Lett. 2002, 81, 2403.
[7] a) R. L. Fu, S. Xu, Y. N. Lu, J. J. Zhu, Cryst. Growth Des. 2005, 5, 1379; b) W. Z. Wang, B. Poudel, Y. Ma, Z. F. Ren, J. Phys. Chem. B 2006, 110, 25702.
[8] a) L. Li, Y. Zhang, G. Li, L. Zhang, Chem. Phys. Lett. 2003, 378, 244; b) J. Heremans, C. M. Thrush, Y. M. Lin, S. Cronin, Z. Zhang, M. S. Dresselhaus, J. F. Mansfield, Phys. Rev. B 2000, 61, 2921; c) J. Heremans, C. M. Thrush, Z. Zhang, X. Sun, M. S. Dresselhaus, J. Y. Ying, D. T. Morelli, Phys. Rev. B 1998, 58, R10091.
[9] a) Y. Gao, H. Niu, C. Zeng, Q. Chen, Chem. Phys. Lett. 2003, 367, 141; b) Y. Xu, Z. Ren,W. Ren, G. Cao, K. Deng, Y. Zhong, Nanotechnol. 2008, 19, 115602; c) P. Chiu, I. Shih, Nanotechnol. 2004, 15, 1489; d) G. Bhimarasetti, M. K. Sunkara, J. Phys. Chem. B 2005, 109, 16219.





