Publications
Cover Gallery
 |
 |
 |
 |
Publications
32. "Differentially Protected Glycols from α-Halo Boronic Esters and Lithiated Benzoates" K. Bojaryn, C. Hirschhäuser* Eur J. Org. Chem. 2024, 27, e202300953. Highlighted on the Front Cover
31. "A unified Strategy for the synthesis of aldohexoses by boronate assisted assembly of CH2X2 derived C1-building blocks" S. Kirupakaran, C. Hirschhäuser* Chem. Sci. 2023, 14, 9838-9842.
30. "A Fluorophore-Labeled Lysine Dendrimer with an Oxo-Anion-Binding Motif for Tracking Gene Transfection” N. Aldemir, C. Vallet, S, S. K. Knauer, C. Schuck, C. Hirschhäuser* ChemBioChem 2023, 10.1002/cbic.202300296.
29. "Direct Radical C1 Homologations” C. Hirschhäuser, H.-G. Korth, Homologation Reactions, Weinheim: Wiley VCH, ISBN: 978-3-527-34815-2, 2023, 759-784.
28. "Total Synthesis of Resveratrone and iso-Resveratrone” S. Fritsch, N. Aldemir, J. Balszuweit, K. Bojary, J. Voskuhl, C. Hirschhäuser* ChemistryOpen 2022, 11, e202200098. Highlighted in Chemistry Views
27. "Cover-Profile: Advances towards Cell-Specific Gene Transfection: A Small-Molecule Approach Allows Order-of-Magnitude Selectivity“ T. Dirksmeyer, P. Stahl, C. Vallet, S. Knauer, M. Giese, C. Schmuck, C. Hirschhäuser* Chem. Eur. J. 2022, 28, e202202024.
26. "Advances towards Cell-Specific Gene Transfection: A Small-Molecule Approach Allows Order-of-Magnitude Selectivity“ T. Dirksmeyer, P. Stahl, C. Vallet, S. Knauer, M. Giese, C. Schmuck, C. Hirschhäuser* Chem. Eur. J. 2022, 28, e202104618. Highlighted on the Front Cover
25. "Transition Metal Catalyst Free Synthesis of Olefins from Organoboron Derivatives" K. Bojaryn, C. Hirschhäuser*, Chemistry a European Journal, 2022, 28, e202104125. Highlighted by Frontispiece
24. "Supramolecular polymers with reversed viscosity/temperature profile for application in motor oils" J.-E. Ostwaldt, C. Hirschhäuser, S. K. Maier, C. Schmuck, J. Niemeyer*, Beilstein Journal of Organic Chemistry, 2021,17, 105-114.
23. "A Metallosupramolecular Coordination Polymer for the ‘Turn‐on’ Fluorescence Detection of Hydrogen Sulfide" J. Hatai, C. Hirschhäuser, C. Schmuck, J. Niemeyer*, ChemistryOpen, 2020, 9, 786-792.
22. "Dual pH-Induced Reversible Self-Assembly of Gold Nanoparticles by Surface Functionalization with Zwitterionic Ligands" H.B. He, J. E. Oswaldt, C. Hirschhäuser, C. Schmuck, J. Niemeyer*, Small, 2020, 28, 2001044.
21. "Multi-Stimuli-Responsive Supramolecular Polymers Based on Noncovalent and Dynamic Covalent Bonds" J. Hatai*, C. Hirschhäuser, J. Niemeyer*, C. Schmuck, ACS Appl. Mater. Interfaces, 2020, 12, 2107-2115.
20. "Cancer‐Cell Specific Drug Delivery by a Tumor Homing CPP‐Gossypol Conjugate Employing a Tracelessly Cleavable Linker" S. K. Maity, P. Stahl, A. Hensel, S. Knauer, C. Hirschhäuser*, C. Schmuck, Chemistry - A European Journal, 2020, 26, 3010-3015. Highlighted by Cover Feature
19. "A Selective Cucurbit[8]uril‐Peptide Beacon Ensemble for Ratiometric Fluorescence Detection of Peptides" D. Maity, K. I. Assaf, W. Sicking, C. Hirschhäuser*, W. M. Nau, C. Schmuck, Chemistry - A European Journal, 2019, 25, 13088-13093.
18. "Non-viral transfection vectors: are hybrid materials the way forward?" A. Gigante*, M. Li, S. Junghänel, C. Hirschhäuser, S. Knauer, C. Schmuck*, MedChemComm, 2019, 10, 1692-1718.
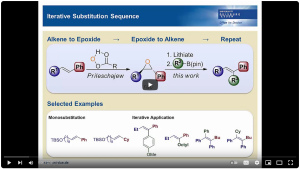
17. "Iterative Synthesis of Alkenes by Insertion of Lithiated Epoxides into Boronic Esters"; K. Bojaryn, S. Fritsch, C. Hirschhäuser*, Organic Letters, 2019, 21, 2218-2222.
16. "A Branched Tripeptide with an Anion‐Binding Motif as a Novel Delivery Carrier for Efficient Gene Transfection"; H. Jiang, Xiao-Yu Hu, S. Mosel, C. Hirschhäuser, S. K. Knauer, C. Schmuck, ChemBioChem, 2019, 20, 1410-1416
15. "A Complementary Toolbox of Iterative Methods for the Stereoselective Synthesis of Heteroatom-Rich Motives from C1-Building Blocks"; S. Kirupakaran, H.-G. Korth, C. Hirschhäuser*, Synthesis, 2018, 50, 2307-2322.
14. "Ambient-Pressure Asymmetric Preparation of S,S-DICHED, a C2-Symmetrical Director for Matteson Reactions"; K. Bojaryn, C. Hoffmann, F. R. Struth, C. Hirschhäuser*, Synlett, 2018, 29, 1092-1094. Highlighted in Synform
13. "Screencasts als unterstützendes Mittel im Chemiepraktikum"; C. Hirschhäuser,* M. Giese, C. Schmuck, Flexibles Lernen mit digitalen Medien ermöglichen, Münster: Waxmann, ISBN: 978-3-8309-3652-7, 2018, 264-283.
12. "Protein Surface Recognition by Synthetic Molecules"; K. Samanta, P. Jana, C. Hirschhäuser and C. Schmuck*, Comprehensive Supramolecular Chemistry II, 2017, 4, 295–349.
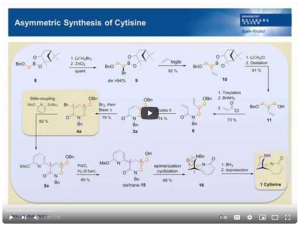
11. “A Modular Approach to the Asymmetric Synthesis of Cytisine”; F. R. Struth, C. Hirschhäuser*; European Journal of Organic Chemistry, 2016, 2016, 958-964.
10. "Organometallic nucleosides induce non-classical leukemic cell death that is mitochondrial-ROS dependent and facilitated by TCL1-oncogene burden"; C. Prinz, E. Vasyuntina, G. Lohmann, A. Schrader, S. Romanski, C. Hirschhäuser, P. Mayer, C. Frias, C. D. Herling, M. Hallek, H. G. Schmalz, A. Prokop, D. Mougiakakos, M. Herling*; Molecular Cancer, 2015, 14:114.
9. “Synthesis of Mono- and Diaza-‘Pyridones’ via Stille Coupling of Alkoxystannanes”; C. Smith, C. Hirschhäuser G. Malcolm, D. Nasrallah, T. Gallagher*; Synlett, 2014, 25, 1904-1908.
8. „Nucleoside Analogues with a 1,3‐Diene-Fe(CO)3 Substructure: Stereoselective Synthesis, Configurational Assignment, and Apoptosis‐Inducing Activity”; C. Hirschhäuser, J. Velcicky, D. Schlawe, E. Hessler, A. Majdalani, J.-M. Neudörfl, A. Prokop*, T. Wieder, H.-G. Schmalz*, Chemistry – A European Journal, 2013, 19, 13017-13029.
7. „Spiro-fused Pyrrolidine, Piperidine, and Oxindole Scaffolds from Lactams”; C. Hirschhäuser, J. Parker, M. Perry, T. Gallagher*, Organic Letters, 2012, 14, 4846-4849.
6. „1,2-Dihydro-4,6-dimethyl-2-thioxo-3-Pyridinecarbonitrile – First Update”; C. Hirschhäuser, Electronic Encyclopedia of Reagents for Organic Synthesis (eEROS), 2012.
5. „Paraoxonase-1 and clopidogrel efficacy reply”; H. Bouman, E. Schömig, J. Werkum, J. Velder, C. Hackeng, C. Hirschhäuser, C. Waldmann, H.-G. Schmalz, J. Berg, D. Taubert*, Nature Medicine, 2011, 17, 1042-1044.
4. „Core Modifications of Cytisine. A Modular Synthesis”; C. Hirschhäuser, C. Haseler, T. Gallagher*, Angew. Chem., 2011, 123, 5268-5271; Angew. Chem. Int. Ed., 2011, 50, 5162‑5165.
3. „Paraoxonase-1 is the Major Determinant of Clopidogrel Efficacy – a Mechanism-Based Case-Cohort Study”; H. Bouman, E. Schömig, J. Werkum, J. Velder, C. Hackeng, C. Hirschhäuser, C. Waldmann, H.-G. Schmalz, J. Berg, D. Taubert*, Nature Medicine, 2011, 17, 110-116.
2. „Intramolecular 1,6-Addition to 2-Pyridones, Scope and Mechanism”; T. Gallagher*, I. Derrick, P. Durkin, C. Haseler, C. Hirschhäuser, P. Magrone, J. Org. Chem., 2010, 75, 3766‑3774.
1. „A Scalable Synthesis of (±)-2-Oxoclopidogrel”; J. Velder, C. Hirschhäuser, C. Waldmann, D. Taubert, H. Bouman, H.-G. Schmalz*, Synlett, 2010, 21, 467-470.