Experiment Electrochemistry 2Redox reactions
Introduction to cyclovoltammetry
For an initial introduction to cyclovoltammetry, consider checking out this Wikipedia article.
This experiment introduces you to the electrochemical three-electrode setup in a Schott glass. Using a classical electrochemical redox reaction $\mathrm{Fe^{3+} / ,Fe^{2+} }$, you will learn about the dependence of electrochemical charge transfer processes on the potential of the working electrode. Please start by reading the article by Elgirishi . I couldn't write it better myself. The article also specifically addresses the different representations of redox reactions in the USA and the rest of the world.
The Ag/AgCl reference electrode
You will be using the reference electrode. Read some text concerning this electrode.

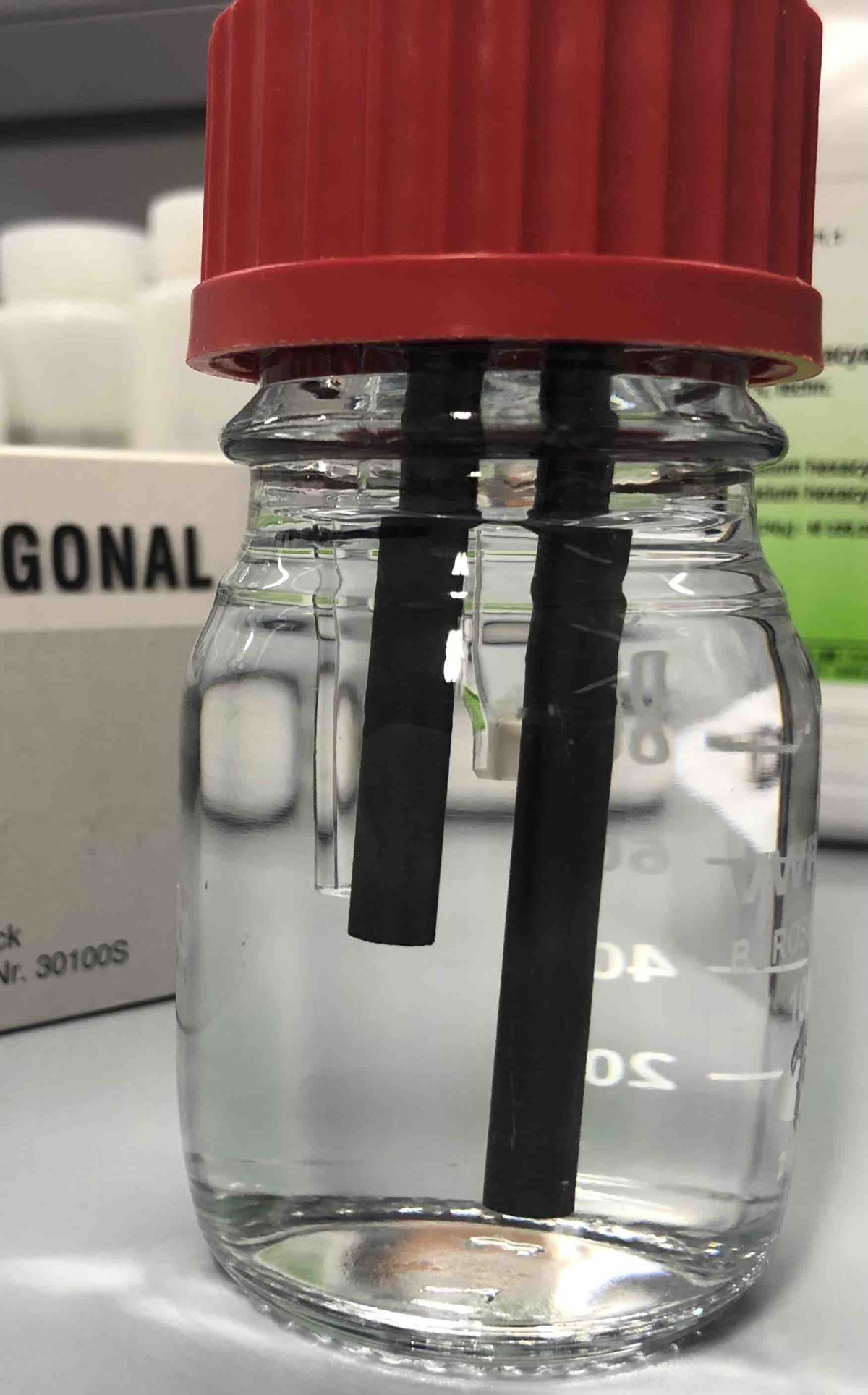
Preparing graphite rodes
Before using graphite rods it is better to cook them in boiling Millipore water as it is shown on the left side. Please observe the formation of gas bubbles on the graphite rods when heating the water. Why do these bubbles form?

This is the electrochemical cell from below. You can see the two graphite rods and the frit for the use of the reference electrode.
Discussion of your results:
- For a detailed discussion of the cyclovoltammogramms please read the paper of Kissinger .
- If you are interested in analytical electrochemistry you can read this work.
Requirements for entering the lab:
- Geometry of cylinder rods
- Graphite and other carbons
- Please explain the basics of cyclovoltammetry to your collegue.
- Have a look to your sciebo folder.
- Try to unterstand the Igor procedure file. Visual Studio Pro has an Igor Pro utility. Download it. If you're unable to locate it, please take a moment to check here.
Redox electrochemistry in our lab
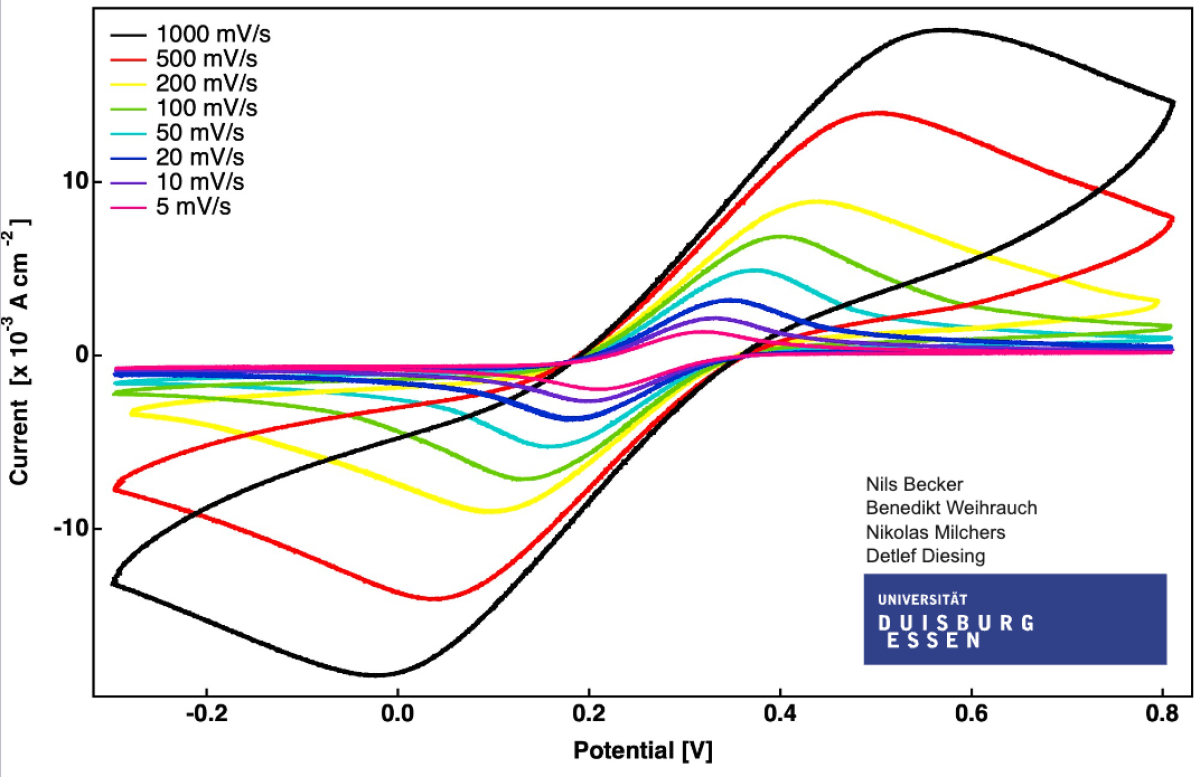
Cyclovoltammograms of an Fe redox couple
These are results from your colleagues. Have a look over the really remarkable range of scan rates. Do you have an idea about the preak separation of the anodic and the cathodic current maximum ?
If you want now to discuss in detail with the preciseness of a scalable vector graphics press here. Happy Zooming !!
Comparison: With and without redoxactive species
With and without Fe species
In this paragraph you find a comparison of these electrolytes
- 1M KNO3 electrolyte
- 1M KNO3 + 5mM Fe2+ +5mM Fe3+ electrolyte

