Kinetics
The Kinetics team studies the chemical kinetics and mechanisms of fast high-temperature reactions in the gas phase. A central aspect is the elucidation of complex reaction networks that play key roles in the following areas: ignition and (partial) oxidation of conventional and novel fuels, formation of pollutants (such as soot) during practical combustion processes, and the tailored synthesis of functional nanomaterials from the gas phase.
...
In addition, the group investigates ignition properties of liquid and gaseous fuels over a wide range of conditions, including high pressure. By transferring the detailed kinetics and mechanistic information via chemical kinetics models to complex practical environments such as engines or flame reactors, the team contributes to improving the understanding and the performance of those processes.
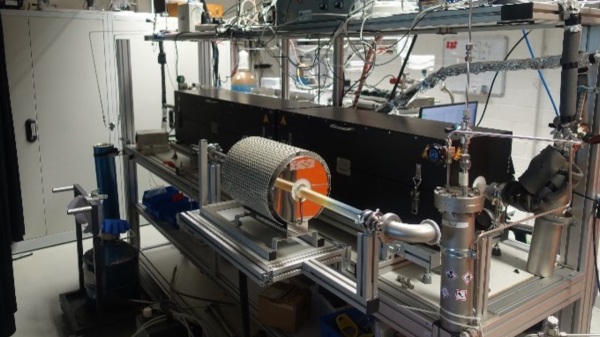
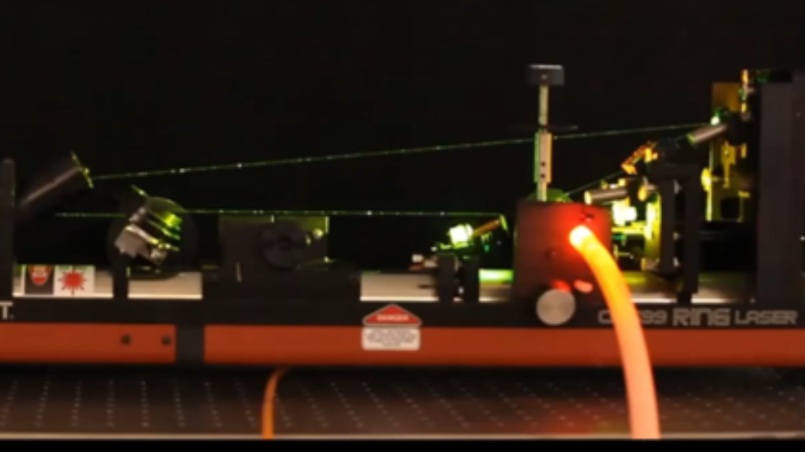
Five complementary shock-tube facilities are used to carry out the experimental work. In these setups, gas mixtures can be heated to well-defined temperatures between 600 and 5000 K within a few microseconds. This temperature increase initiates chemical reactions, that are monitored using a wide variety of modern detection techniques. With their high sensitivity and selectivity, optical (including laser-based) diagnostics are employed for the time-resolved detection of individual species, including reactive intermediates. High-repetition-rate mass spectrometry enables the time-resolved, simultaneous detection of multiple species and gas chromatography is applied for the quantitative product analysis in complex reaction systems.
The group also applies quantum chemical methods and statistical rate theory to get further insight into reaction mechanisms and to calculate accurate temperature- and pressure dependent rate coefficients. These theoretical methods complement the experimental analysis and extend parameter range to conditions that are not accessible experimentally.