GRK 2762 - Research Projects
Project L2Subtype-specific vulnerabilities of KRAS-driven NSCLC cells with intrinsic or adaptive radiation resistance
Principal Investigator
PD Dr. rer. nat. Johann Matschke
Institute of Cell Biology (Cancer Research)
Universitätsklinikum Essen
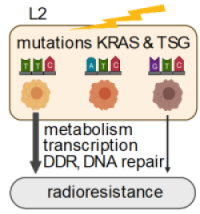
Summary
Clinically relevant co-occurring somatic mutations in tumor suppressor genes STK11/LKB1, KEAP1 emerged as potential biomarkers for an increased risk of radiotherapy (RT) failure in KRAS-driven lung cancer. Own preliminary work revealed that in addition to oxidative stress and DNA damage, ionizing radiation (IR) induces severe energy stress in cancer cells. In this context, oncogenic KRAS mutations accelerated metabolic recovery and DNA damage repair. We thus speculate that to maintain survival upon RT cancer cells have to balance protection against oxidative and energy stress with their needs in energy and metabolites for the repair of radiation-induced lethal DNA double-strand breaks (DSBs). The proposed project aims to define the impact of the molecular background defined by clinically relevant co-occurring somatic mutations on the capacity of KRAS-driven lung cancer cells to dynamically adapt their transcriptional programs and metabolic phenotypes in support of DSB repair and survival upon exposure to IR and perturbation by distinct environmental conditions.
Selected Publications
Krysztofiak A, Szymonowicz K, Hlouschek J, Xiang K, Waterkamp C, Larafa S, Goetting I, Vega-Rubin-de-Celis S, Theiss C, Matschke V, Hoffmann D, Jendrossek V, Matschke J. Metabolism of cancer cells commonly responds to irradiation by a transient early mitochondrial shutdown. iScience. 2021;24(11):103366.
Matschke J, Larafa S, Jendrossek V. Metabolic reprograming of antioxidant defense: a precision medicine perspective for radiotherapy of lung cancer? Biochem Soc Trans. 2021.
Hlouschek J, Hansel C, Jendrossek V, Matschke J. The Mitochondrial Citrate Carrier (SLC25A1) Sustains Redox Homeostasis and Mitochondrial Metabolism Supporting Radioresistance of Cancer Cells With Tolerance to Cycling Severe Hypoxia. Front Oncol. 2018;8:170.
Hlouschek J, Ritter V, Wirsdorfer F, Klein D, Jendrossek V, Matschke J. Targeting SLC25A10 alleviates improved antioxidant capacity and associated radioresistance of cancer cells induced by chronic-cycling hypoxia. Cancer Lett. 2018;439:24-38.
Matschke J, Riffkin H, Klein D, Handrick R, Ludemann L, Metzen E, Shlomi T, Stuschke M, Jendrossek V. Targeted Inhibition of Glutamine-Dependent Glutathione Metabolism Overcomes Death Resistance Induced by Chronic Cycling Hypoxia. Antioxid Redox Signal. 2016;25(2):89-107.
Matschke J, Wiebeck E, Hurst S, Rudner J, Jendrossek V. Role of SGK1 for fatty acid uptake, cell survival and radioresistance of NCI-H460 lung cancer cells exposed to acute or chronic cycling severe hypoxia. Radiat Oncol. 2016;11:75.
Shannan B, Matschke J, Chauvistre H, Vogel F, Klein D, Meier F, Westphal D, Bruns J, Rauschenberg R, Utikal J, Forschner A, Berking C, Terheyden P, Dabrowski E, Gutzmer R, Rafei-Shamsabadi D, Meiss F, Heinzerling L, Zimmer L, Livingstone E, Varaljai R, Hoewner A, Horn S, Klode J, Stuschke M, Scheffler B, Marchetto A, Sannino G, Grunewald TGP, Schadendorf D, Jendrossek V, Roesch A. Sequence-dependent cross-resistance of combined radiotherapy plus BRAF(V600E) inhibition in melanoma. Eur J Cancer. 2019;109:137-153.
Xiang K, Jendrossek V, Matschke J. Oncometabolites and the response to radiotherapy. Radiat Oncol. 2020;15(1):197.


