Science: Research group Immunology Sepsis/Trauma
Reprogramming of DCs during polymicrobial sepsis
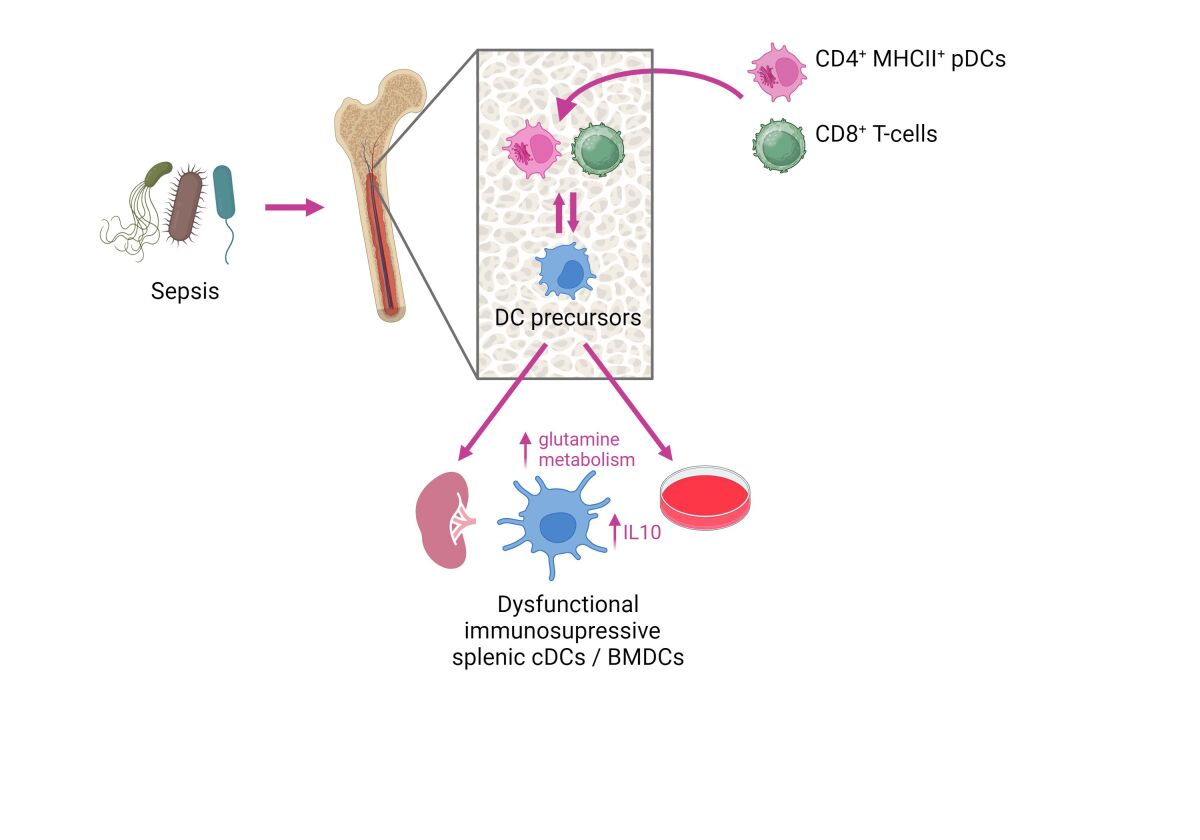
Dendritic cells (DCs) coordinate the innate and adaptive immune responses against pathogens and largely contribute in sepsis-related immunosuppression. Using a clinically relevant sepsis model, we have demonstrated that sepsis exerts a long-lasting effect on DC precursors and modulate their differentiation in the bone marrow. This leads to the development of DCs with an immunosuppressive phenotype that is reflected by an elevated production of the anti-inflammatory cytokine IL-10 and inhibition of protective Th1-type immune responses (Flohé SB et al J Leukoc Biol 2006). Moreover, newly generated DCs from bone marrow directly impair the immune defense against P. aeruginosa, a frequent cause of nosocomial infection, in the lung (Pastille E J Immunol 2011).
In search of the mechanisms underlying the modulation of DC differentiation we have identified several cell populations which seem to contribute to a reprogramming of DC precursors such as activated CD4+ MHCII+ plasmacytoid DCs (Smirnov A, Front Immunol 2017) and CD8+ T-cells (Antoni A-C, Front Immunol 2022). In addition, the functional reprogramming of DCs during sepsis is associated with an altered glutamine metabolism of so far unknown origin.
Our ongoing work focuses on the molecular mechanisms responsible for the long-lasting modulation of DC precursors in sepsis. The restoration of DC function might represent novel targets for the development of immunotherapy in sepsis.
Origin of NK cell dysfunction in sterile inflammation and sepsis
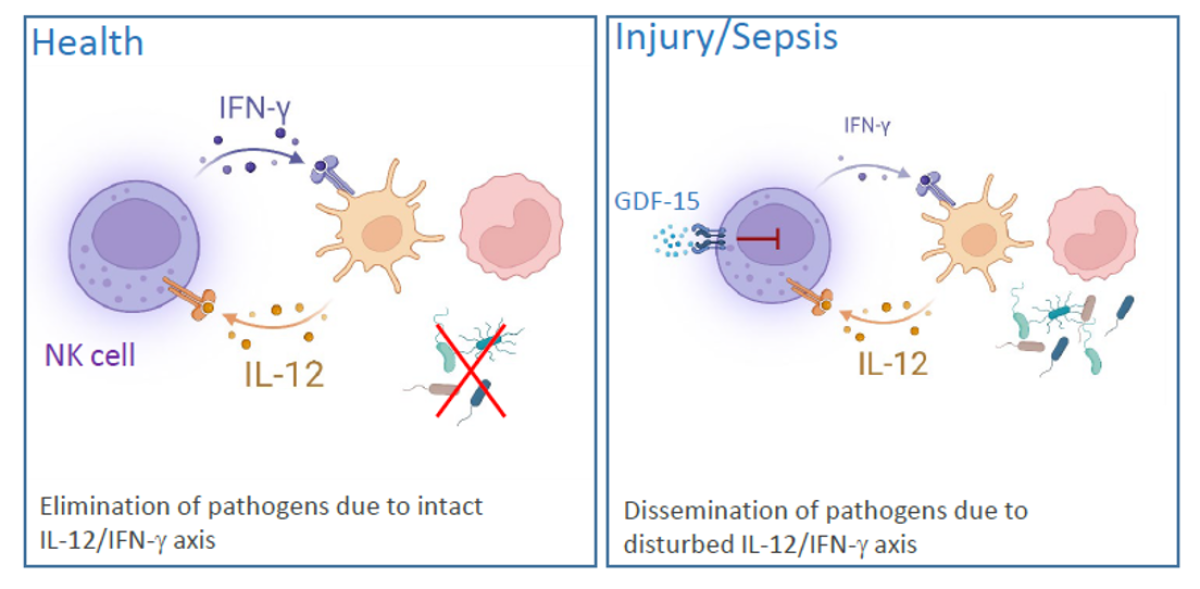
Dendritic cells (DCs) that reside in almost all tissues detect invading pathogens and secrete diverse cytokines and chemokines that recruit and activate further immune cells. Interleukin (IL) 12 that is released by DCs during infection activate Natural Killer (NK) cells. In response to IL-12 NK cells produce IFN-γ that supports the elimination of pathogens by macrophages and neutrophils. Our previous work has shown that NK cells are almost completely impaired in IFN-γ synthesis within 24 h after severe traumatic injury. The inhibition of NK cells is long-lasting and still evident at discharge from the hospital, often months after injury. We have identified a disturbed IL-12/IFN-γ axis in the patients’ NK cells that is mediated by circulating growth/differentiation factor (GDF) 15 and endogenous reprogramming of cell function (Kleinertz et al 2019, EBioMedicine 43:380). The degree of NK cell dysfunction is related to the severity of tissue injury (Müller et al. 2021, Life 12:13.) and is associated with changes in cell metabolism.
Using a model for polymicrobial sepsis we found evidence for a disturbed interaction of DCs and NK cells in the lung (Pastille et al. 2015 Innate Immun. 21:115-26) indicating that NK cell dysfunction is not restricted to sterile systemic inflammation but also develops in systemic infection. Current work focuses on the molecular mechanisms underlying the disturbed IL-12/IFN-γ axis that might represent therapeutic targets to restore the function of NK cells.