ICCE - Applications
Transmitted Light Techniques
label-free contrast modes used in optical microscopy
Brightfield microscopy
Is the simplest microscopy technique where the light is transmitted through the specimen, making it appear dark against an illuminated white background. As the optics to not change the colour of the specimen, it can be used to visualise stained structures within a specimen.
Phase contrast
The phase contrast technique converts phase shifts, caused by small density differences of the sample structures, into amplitude differences that are detectable by the human eye (bright-dark-contrasts). It is the easiest and most common way to image biological samples.
Differential Interference Contrast (DIC) microscopy
In DIC microscopy only polarized light is used for specimen illumination. This technique makes use of gradients in the optical path length and phase shifts to make phase objects visible. In this way it is possible to observe living cells and organisms with adequate contrast and resolution under the light microscope.
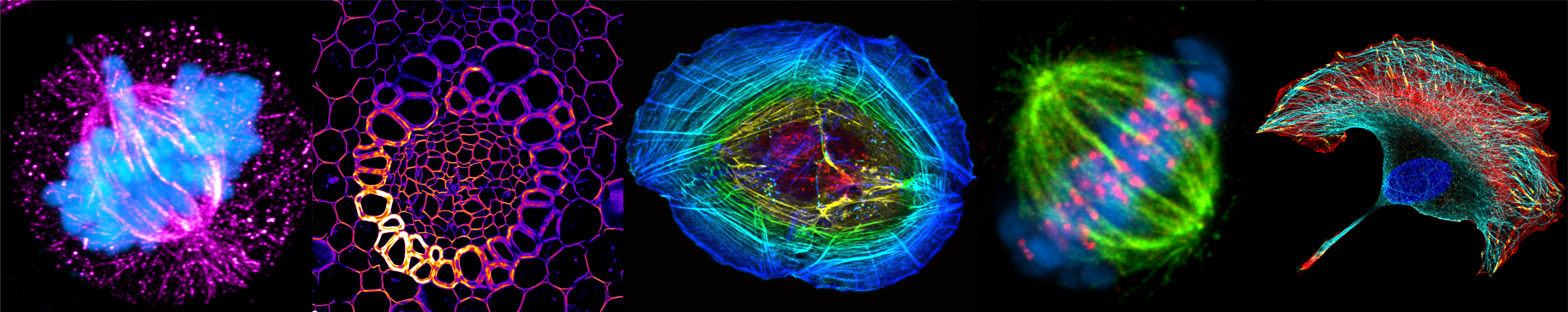
Fluorescence microscopy
Helps to overcome the limitations of ordinary light microscopy by combining magnifying properties of light microscopy with fluorescence visualization. Both, autofluorescence of the specimen or fluorescent dyes can be used to visualise cell structures or processes.
Total Internal Reflection Fluorescence Microscopy (TIRF)
TIRF microscopy offers an outstanding signal-to-background ratio as the out-of-focus fluorescence is minimized due to the low penetration depth of the employed evanescent field. Additionally, production of harmful oxygen and therefore phototoxic stress for cells is greatly reduced as only a portion of the cell is exposed to the energy of the evanescent field. It can be combined with FRET, FRAP and other techniques. Note: Only adherent cultured cells can be used for experiments.
Fluorescence Lifetime Imaging (FLIM)
FLIM as a fluorescence lifetime based imaging technique does not depend on concentration, absorption by the sample, sample thickness, photo-bleaching and/or excitation intensity and is hence more robust than intensity based methods. Additional information can be obtained as the fluorescence lifetime depends on environmental parameters such as pH, ion or oxygen concentration, molecular binding or the proximity of energy acceptors.
Fluorescence Recovery After Photobleaching (FRAP)
FRAP can be used to analyse the mobility of molecules within living cells. Fluorescent molecules in a region of interest (ROI) are photobleached by a short pulse of high intensity laser light. The recovery rate of the fluorescence can be used to obtain information on active transport, diffusion and binding properties of the analysed molecules.
Förster Resonance Energy Transfer (FRET)
FRET is a non-radiative energy transfer between a suitable donor and acceptor fluorophore pair. As the efficiency of this transfer is distance depend, it can be used to detect molecule-molecule interactions in the nanometer range (< 10nm).
sensitized emission
Is commonly used to employ FRET as an imaging technique on a widefield or confocal microscope. To analyse distances of the FRET pairs the donor fluorophore is excited by a specific wavelength, and the signal is collected by using suitable emission filters for the fluorescence of the FRET pair. Due to the spectral overlap of most FRET pairs, calculation of quantitatively accurate FRET efficiencies is complicated and requires extensive control experiments.
acceptor photobleaching
Combining sensitized emission with acceptor photobleaching enables the calculation of the apparent FRET efficiency. Photobleaching of the acceptor in fixed samples leads to unquenching of the donor fluorescence. The donor signal in the prebleached and postbleached images can then be used to calculated the apparent FRET efficiency.
FLIM-FRET
FLIM-FRET monitors changes in lifetime values of the donor without and with the acceptor, as the occurrence of FRET leads to a shortening of the donor lifetime. FLIM-FRET provides high temporal resolution of protein-protein interactions in live specimens and is ideal for dark acceptors and the investigation of NADH molecules such as NADH, FAD, Tryptophan, etc.
Fluorescence (Cross-) Correlation Spectroscopy (F(C)CS)
Fluorescence correlation spectroscopy (FCS) can be used to analyze concentrations and mobilities of molecules. It exploits fluorescence fluctuations induced by low numbers of diffusing labeled particles in a confocal setup. Fluorescence autocorrelation spectroscopy determines diffusion coefficients within short acquisition times (usually tens of seconds) and with excellent statistics. Compared with FRAP, much lower probe concentrations and laser powers are used, minimizing the perturbation to the biological system. Its dual-color variant, FCCS, can be used to monitor molecular interactions and enzymatic reactions as well as dynamic colocalization, for example, of cargo in small vesicles.
Super-Resolution Microscopy
Optical techniques used to resolve structures beyond the diffraction-limited resolution of conventional light microscopy.
Structured Illumination Microscopy (SIM)
SIM is a laser-based wide-field microscopy technique with the addition of a movable diffraction grating to enhance spatial resolution. The created stripe pattern of light generates the so-called Moiré fringes with the sample. Using special software the final super-resolved image (SR-image) is reconstructed from several raw images, each being acquired at different orientation of the structured illumination. Advantages of SIM are:
- 2-fold increase in spatial resolution over wide-field microscopy,
- 4D imaging at fast frame rate,
- thicker sections can be imaged,
- no fluorophores need but image quality increases with bright and photostable dyes as well as precise targeting.

