ZMB Member Hemmo Meyer
ZMB Member
Hemmo Meyer
Next ZMB-Member
Prof. Dr. Hemmo Meyer
Group
Molecular Biology ICenter of Medical Biotechnology (ZMB)
Faculty of Biology
University of Duisburg-Essen
Universitätsstr. 2
45141 Essen
- +49 201 183 4217
- Website
- Speaker CRC 1430
- Press Releases
- Selected Publications
- ORCID ID
- Publication Metrics
-

- ZMB Research Program
Molecular and Chemical Cell Biology
Research Overview
Cellular homeostasis, proliferation and stress responses
Cells need to cope with a multitude of stress conditions that relentlessly inflict damage to its most vital components. This includes insults to the DNA that threatens genome stability, damage of proteins that can then form toxic aggregates, or injury of whole organelles such as mitochondria and lysosomes that releases harmful components. Cells have developed sophisticated molecular responses to these stresses that maintain protein homeostasis and organelle function, and ensure genomic stability. We are interested in deciphering these responses and uncover how they counteract stress-induced cell death and aging-related degeneration, or maintain cell proliferation.
The ubiquitin-proteasome system (UPS) is critically involved in cellular stress responses. Ubiquitination triggers degradation of damaged proteins by the proteasome, or removal of protein aggregates or hole organelles in the lysosome through autophagy. In addition, it regulates signalling pathways such as the DNA damage response and coordinates them with cell cycle progression.
A focus of our research has been the AAA+-type ATPase VCP/p97, which has emerged as a pivotal element of the UPS. It governs a variety of processes such as ER-associated degradation, ribosomal quality control, DNA damage responses as well as autophagy. Mutations in VCP/p97 in humans cause degenerative diseases including amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), while pharmacological inhibition of VCP/p97 is considered as a strategy in cancer therapy. We have been working to reveal the molecular function of VCP/p97 and to understand how it cooperates with a host of accessory factors to trigger diverse stress responses in different compartments.
Keywords:
Cellular stress responses, proteostasis, ubiquitin-proteasome system, autophagy, lysosomal membrane permeabilization, lysophagy, mitophagy, DNA damage response, double strand break repair, cell cycle regulation.
Distribution of published plasmids through addgene.org.
Press Releases
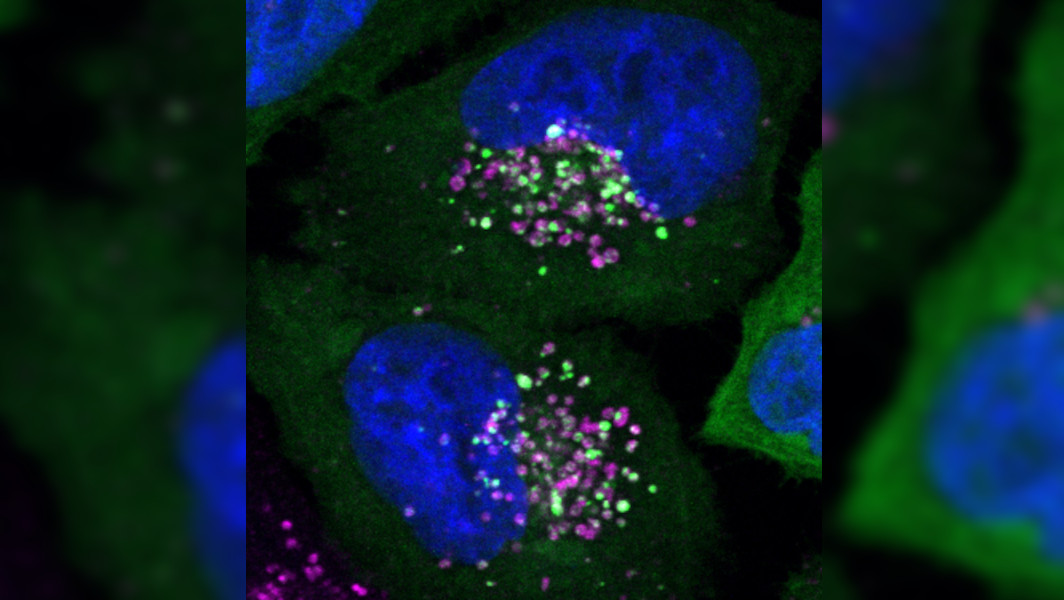
Decomposition of Damaged Lysosomes New Signaling Pathway Decoded
[08.04.2024] Lysosomen sind die Recyclinghöfe in unseren Zellen: Die kugelförmigen Organellen bauen in ihrem Innern sowohl körpereigene als auch Fremdstoffe ab – zur anschließenden Weiterverwertung. Da ihr Inneres einen sauren pH-Wert aufweist, sind Schäden in der Membran, die die Lysosomen umschließt, gefährlich für Zellen. Wissenschaftler:innen der UDE haben einen neuen Signalweg identifiziert, der in diesem Fall zum Abbau des Lysosoms führt. Ihre Erkenntnisse, veröffentlicht in Molecular Cell, könnten dazu beitragen, neue Ansätze für die Behandlung neurodegenerativer Krankheiten zu entwickeln.

Catabolic Processes in Cells Controlling the Danger Within
[06.07.2022] Trillions of cells in our body work non-stop to keep us alive. This generates waste that is decomposed in specialized cellular organs. But what happens if the cellular trash cans don't work? Researchers assume that this is the cause of numerous diseases. Biologists from UDE, together with a team from Munich, have now been able to show how cells protect themselves from their defective trash cans – because their contents are pretty serious.
Selected Publications
-
Alternating binding and p97-mediated dissociation of SDS22 and I3 recycles active PP1 between holophosphatasesIn: Proceedings of the National Academy of Sciences of the United States of America (PNAS) Vol. 121 (2024) Nr. 36, e2408787121Online Full Text: dx.doi.org/ (Open Access)
-
Lysosomal damage sensing and lysophagy initiation by SPG20-ITCHIn: Molecular Cell (2024) pp. 1556 - 1569.e10Online Full Text: dx.doi.org/ Online Full Text (Open Access)
-
The Endo-Lysosomal Damage ResponseIn: Annual Review of Biochemistry Vol. 93 (2024) Nr. 1, pp. 367 - 387Online Full Text: dx.doi.org/ (Open Access)
-
Ubiquitin profiling of lysophagy identifies actin stabilizer CNN2 as a target of VCP/p97 and uncovers a link to HSPB1In: Molecular Cell Vol. 82 (2022) Nr. 14, pp. 2633 - 2649.e7Online Full Text: dx.doi.org/ (Open Access)
-
Ubiquitin-Independent Disassembly by a p97 AAA-ATPase Complex Drives PP1 Holoenzyme FormationIn: Molecular Cell Vol. 72 (2018) Nr. 4, pp. 766 - 777Online Full Text: dx.doi.org/ (Open Access)
-
VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and SignalingIn: Molecular Cell Vol. 69 (2018) Nr. 2, pp. 182 - 194Online Full Text: dx.doi.org/ (Open Access)
-
Detection and Clearance of Damaged Lysosomes by the Endo-Lysosomal Damage Response and LysophagyIn: Current Biology Vol. 27 (2017) Nr. 24, pp. R1330 - R1341Online Full Text: dx.doi.org/
-
VCP/p97 Extracts Sterically Trapped Ku70/80 Rings from DNA in Double-Strand Break RepairIn: Molecular Cell Vol. 64 (2016) Nr. 1, pp. 189 - 198Online Full Text: dx.doi.org/ (Open Access)

